Copyright Michael D. Robbins 2005
Astro-Rayological
Interpretation & Charts
Quotes
Biography
Images Physiognomic Interpretation
An expert is a man who has made all the mistakes which can be made in a very narrow field.
An expert is someone who knows some of the worst mistakes, which can be made, in a very narrow field.
How wonderful that we have met with a paradox. Now we have some hope of making progress.
If anybody says he can think about quantum physics without getting giddy, that only shows he has not understood the first thing about them.
If quantum mechanics hasn't profoundly shocked you, you haven't understood it yet.
Never express yourself more clearly than you are able to think.
No, no, you're not thinking; you're just being logical.
Prediction is very difficult, especially if it's about the future.
Technology has advanced more in the last thirty years than in the previous two thousand. The exponential increase in advancement will only continue. Anthropological Commentary The opposite of a trivial truth is false; the opposite of a great truth is also true.
There are some things so serious you have to laugh at them.
We are all agreed that your theory is crazy. The question which divides us is whether it is crazy enough to have a chance of being correct. My own feeling is that it is not crazy enough.
Your theory is crazy, but it's not crazy enough to be true.
The opposite of a fact is falsehood, but the opposite of one profound truth may very well be another profound truth. There are trivial truths and the great truths. The opposite of a trivial truth is plainly false. The opposite of a great truth is also true.
The opposite of a correct statement is a false statement. But the opposite of a profound truth may well be another profound truth.
The opposite of a fact is falsehood, but the opposite of one profound truth may very well be another profound truth."
Two sorts of truth: trivialities, where opposites are obviously absurd, and profound truths, recognized by the fact that the opposite is also a profound truth."
"The opposite of a correct statement is a false statement. But the opposite of a profound truth may well be another profound truth."
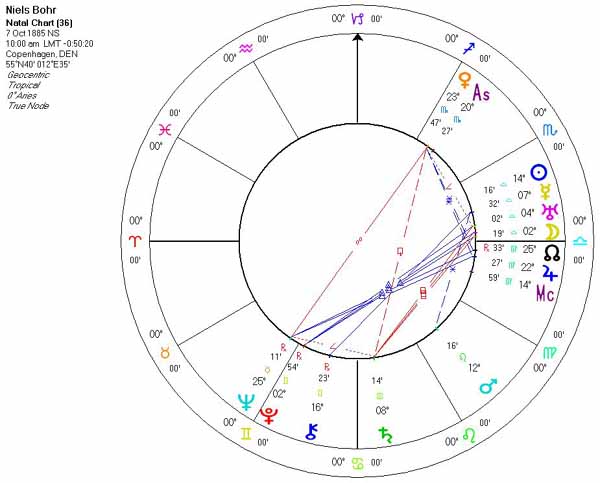
Niels Henrik David Bohr was born in Copenhagen on October 7, 1885, as the son of Christian Bohr, Professor of Physiology at Copenhagen University, and his wife Ellen, née Adler. Niels, together with his younger brother Harald (the future Professor in Mathematics), grew up in an atmosphere most favourable to the development of his genius - his father was an eminent physiologist and was largely responsible for awakening his interest in physics while still at school, his mother came from a family distinguished in the field of education.
After matriculation at the Gammelholm Grammar School in 1903, he entered Copenhagen University where he came under the guidance of Professor C. Christiansen, a profoundly original and highly endowed physicist, and took his Master's degree in Physics in 1909 and his Doctor's degree in 1911.
While still a student, the announcement by the Academy of Sciences in Copenhagen of a prize to be awarded for the solution of a certain scientific problem, caused him to take up an experimental and theoretical investigation of the surface tension by means of oscillating fluid jets. This work, which he carried out in his father's laboratory and for which he received the prize offered (a gold medal), was published in the Transactions of the Royal Society, 1908.
Bohr's subsequent studies, however, became more and more theoretical in character, his doctor's disputation being a purely theoretical piece of work on the explanation of the properties of the metals with the aid of the electron theory, which remains to this day a classic on the subject. It was in this work that Bohr was first confronted with the implications of Planck's quantum theory of radiation.
In the autumn of 1911 he made a stay at Cambridge, where he profited by following the experimental work going on in the Cavendish Laboratory under Sir J.J. Thomson's guidance, at the same time as he pursued own theoretical studies. In the spring of 1912 he was at work in Professor Rutherford's laboratory in Manchester, where just in those years such an intensive scientific life and activity prevailed as a consequence of that investigator's fundamental inquiries into the radioactive phenomena. Having there carried out a theoretical piece of work on the absorption of alpha rays which was published in the Philosophical Magazine, 1913, he passed on to a study of the structure of atoms on the basis of Rutherford's discovery of the atomic nucleus. By introducing conceptions borrowed from the Quantum Theory as established by Planck, which had gradually come to occupy a prominent position in the science of theoretical physics, he succeeded in working out and presenting a picture of atomic structure that, with later improvements (mainly as a result of Heisenberg's ideas in 1925), still fitly serves as an elucidation of the physical and chemical properties of the elements.
In 1913-1914 Bohr held a Lectureship in Physics at Copenhagen University and in 1914-1916 a similar appointment at the Victoria University in Manchester. In 1916 he was appointed Professor of Theoretical Physics at Copenhagen University, and since 1920 (until his death in 1962) he was at the head of the Institute for Theoretical Physics, established for him at that university.
Recognition of his work on the structure of atoms came with the award of the Nobel Prize for 1922.
Bohr's activities in his Institute were since 1930 more and more directed to research on the constitution of the atomic nuclei, and of their transmutations and disintegrations. In 1936 he pointed out that in nuclear processes the smallness of the region in which interactions take place, as well as the strength of these interactions, justify the transition processes to be described more in a classical way than in the case of atoms (Cf. »Neutron capture and nuclear constitution«, Nature, 137 (1936) 344).
A liquid drop would, according to this view, give a very good picture of the nucleus. This so-called liquid droplet theory permitted the understanding of the mechanism of nuclear fission, when the splitting of uranium was discovered by Hahn and Strassmann, in 1939, and formed the basis of important theoretical studies in this field (among others, by Frisch and Meitner).
Bohr also contributed to the clarification of the problems encountered in quantum physics, in particular by developing the concept of complementarily. Hereby he could show how deeply the changes in the field of physics have affected fundamental features of our scientific outlook and how the consequences of this change of attitude reach far beyond the scope of atomic physics and touch upon all domains of human knowledge. These views are discussed in a number of essays, written during the years 1933-1962. They are available in English, collected in two volumes with the title Atomic Physics and Human Knowledge and Essays 1958-1962 on Atomic Physics and Human Knowledge, edited by John Wiley and Sons, New York and London, in 1958 and 1963, respectively.
Among Professor Bohr's numerous writings (some 115 publications), three appearing as books in the English language may be mentioned here as embodying his principal thoughts: The Theory of Spectra and Atomic Constitution, University Press, Cambridge, 1922/2nd. ed., 1924; Atomic Theory and the Description of Nature, University Press, Cambridge, 1934/reprint 1961; The Unity of Knowledge, Doubleday & Co., New York, 1955.
During the Nazi occupation of Denmark in World War II, Bohr escaped to Sweden and spent the last two years of the war in England and America, where he became associated with the Atomic Energy Project. In his later years, he devoted his work to the peaceful application of atomic physics and to political problems arising from the development of atomic weapons. In particular, he advocated a development towards full openness between nations. His views are especially set forth in his Open Letter to the United Nations, June 9, 1950.
Until the end, Bohr's mind remained alert as ever; during the last few years of his life he had shown keen interest in the new developments of molecular biology. The latest formulation of his thoughts on the problem of Life appeared in his final (unfinished) article, published after his death: "Licht und Leben-noch einmal", Naturwiss., 50 (1963) 72: (in English: "Light and Life revisited", ICSU Rev., 5 ( 1963) 194).
Professor Bohr was married, in 1912, to Margrethe Nørlund, who was for him an ideal companion. They had six sons, of whom they lost two; the other four have made distinguished careers in various professions - Hans Henrik (M.D.), Erik (chemical engineer), Aage (Ph.D., theoretical physicist, following his father as Director of the Institute for Theoretical Physics), Ernest (lawyer).
Niels Bohr died in Copenhagen on November 18, 1962.
Niels Henrik David Bohr (October 7, 1885 – November 18, 1962) was a Danish physicist who made essential contributions to understanding atomic structure and quantum mechanics.
Born in Copenhagen to Christian Bohr and Ellen Adler, Bohr received his doctorate from Copenhagen University in 1911. He then studied under Ernest Rutherford in Manchester, England. Based on Rutherford's theories, Bohr published his model of atomic structure in 1913, introducing the theory of electrons travelling in orbits around the atom's nucleus, the chemical properties of the element being largely determined by the number of electrons in the outer orbits. Bohr also introduced the idea that an electron could drop from a higher-energy orbit to a lower one, emitting a photon (light quantum) of discrete energy. This became the basis for quantum theory.
In 1916, Bohr became a professor at the University of Copenhagen, and director of the newly constructed "Institute of Theoretical Physics" in 1920. In 1922, he was awarded the Nobel Prize in physics for developing the Copenhagen interpretation of quantum mechanics.
Bohr was largely affected by the philosophy of Søren Kierkegaard of sharp sudden "quality" changes and rejection of the continuous changing. These concepts were manifested in Bohr's quantum theory.
Bohr also conceived the principle of complementarity: that items could be separately analyzed as having several contradictory properties. For example, physicists currently conclude that light is both a wave and a stream of particles - two apparently mutually exclusive properties - based on this principle. Bohr also found philosophical applications for this daringly original principle. Albert Einstein much preferred the determinism of classical physics over the probabilistic new physics of Bohr and Max Planck. He and Bohr had good-natured arguments over the verity of this principle throughout their lives. One of Bohr's most famous students was Werner Heisenberg, a crucial figure in the development of quantum mechanics, but also head of the German atomic bomb project.
Niels Bohr and his wife Margrethe had several children, one of whom, Aage Niels Bohr, also became a very successful physicist: like his father, he won a Nobel prize.
1941, during the German occupation of Denmark in World War II, Bohr was visited by Heisenberg in Copenhagen and apparently learned something of the German plans. In 1943 he escaped to Sweden to avoid arrest by the German police, then travelled to London.
He worked at Los Alamos, New Mexico, USA, on the Manhattan Project, where, according to Richard Feynman, he was known by the assumed name of Nicholas Baker for security reasons. However his role in the project was minor. He is quoted as saying "That is why I went to America. They didn't need my help in making the atom bomb." He was seen as a knowledgeable consultant or "father confessor" on the project [1]. After the war he returned to Copenhagen, advocating for a peaceful use of nuclear energy. He died in Copenhagen in 1962.
Heisenberg claimed in an interview after the war, when the author Robert Jungk was working on the book Brighter Than a Thousand Suns, that he had tried to establish a pact with Bohr such that scientists on neither side should help develop the atomic bomb. He also said that the German attempts were entirely focused on energy production, and that his circle of colleagues tried to keep it that way.
When Bohr saw this claim he disagreed wholeheartedly. He said that Heisenberg had indeed let him know in Copenhagen that he was working on an atomic bomb project, and that he thought that Germany would win the war. He dismissed the idea of any pact as an after-the-fact construction. He drafted several letters to inform Heisenberg about this but never sent any of them.
Michael Frayn's play Copenhagen, which ran on Broadway for a time, explores what might have happened at the 1941 meeting between Heisenberg and Bohr.
Niels Bohr was born in Copenhagen on October 7, 1885. He was the older of the two sons born to Christian Bohr and Ellen, née Adler. His father was a Professor of Physiology at Copenhagen University. In his early childhood his father helped to cultivate his interest in physics. Niels attended Gammelholm Grammar School until 1903, when he enrolled in Copenhagen University. It was at Copenhagen University where he met Professor C. Christiansen. In 1909 Bohr earned his Master's degree in Physics, he went on to get his Doctorate degree in 1911.
In his early childhood Bohr was inspired by his teachers and his father to peruse mathematics and physics in 1922 Bohr stated, “My interest in the study of physics was awakened while I was still in school, largely owing to the influence of my father.”(Niels Bohr, 1922)
While still a student, he conducted in his father's laboratory, an experimental investigation of surface tension by using oscillating fluid jets. His work won him the prize offered by the Academy of Sciences in Copenhagen, and was published in the Transactions of the Royal Society, in 1908.
Bohr's later studies became much more theoretical. In fact his doctoral dissertation on “the explanation of the properties of the metals with the aid of the electron theory” was purely theoretical. To this day his dissertation remains a classic on the subject. It was while doing this work that Bohr was first confronted with Planck's quantum theory of radiation.
In 1911, under Sir J.J. Thomson's guidance Bohr assisted in the experimental work going on in the Cavendish Laboratory, and at the same time he continued to pursue his own theoretical studies. In the spring of 1912 he went to work at Professor Rutherford's laboratory in Manchester, where he preformed theoretical work on the absorption of alpha rays, which was later published in the Philosophical Magazine, in 1913. He then went on to study the structure of atoms based on Rutherford's discovery of the atomic nucleus. By introducing conceptions borrowed from Planck’s Quantum Theory, he worked out a structure of an atom, which with later improvements based on Heisenberg’s ideas in 1925, still is used. In recognition of this work Bohr was awarded the Nobel Prize in physics in 1922.
In 1912 Bohr married Margrethe Nørlund. Together they had six sons, of who two died, the other four made distinguished careers in various professions: Hans Henrik who became a doctor, Erik who became a chemical engineer, Aage who followed his father as Director of the Institute for Theoretical Physics, and Ernest who became a lawyer.
In 1916 Bohr was appointed Professor of Theoretical Physics at Copenhagen University, and from 1920 until his death in 1962, he was the head of their Institute for Theoretical Physics.
Bohr's later activities in his Institute were directed toward research on the atomic nuclei, and of their transmutations and disintegrations. In 1936 he pointed out that the transition processes in atomic nuclei could be described in a classical unlike the way that an atom is described. This was in part due to the size of the transition, compared to the vastness of the region that it occurred in. According to this view, a liquid drop would give a very good picture of the nucleus; this was to become the “liquid droplet theory”. In 1939 Hahn and Strassmann used this theory to help further the understanding of nuclear fission when they were working on splitting uranium.
During the Nazi occupation of Denmark in World War II, Bohr fled to Sweden and then on to England and The U.S.A. While in the U.S. he became associated with the Atomic Energy Project, and then in later years devoted his work to the peaceful application of atomic physics. He also was active in the politics that went with the development of atomic weapons. For example on June 9, 1950, He sent a letter to the United Nations advocating full openness between nations.
Niels Henrik David Bohr died in Copenhagen on November 18, 1962.